IMCIVREE has a well-established safety and tolerability profile1,2
Adverse reactions occurring in 3 or more IMCIVREE-treated patients 2 to <6 years of age (N=12)1*
| (%) | |
| Skin hyperpigmentation1† | 83 |
| Injection site reactions2‡ | 67 |
| Vomiting | 58 |
| Nasopharyngitis | 42 |
| Melanocytic nevus3‖ | 33 |
| Fall | 33 |
| Fever | 33 |
| Upper respiratory tract infection | 33 |
| Cough | 25 |
| Diarrhea | 25 |
Adverse reactions occurring in 2 or more IMCIVREE-treated patients 6 years of age and older (N=43)2¶
| (%) | |
| Skin hyperpigmentation# | 63 |
| Injection site reactions** | 51 |
| Nausea | 26 |
| Spontaneous penile erection†† | 25 |
| Vomiting | 19 |
| Diarrhea | 14 |
| Melanocytic nevus‖ | 14 |
| Headache | 7 |
| Skin striae | 7 |
| Aggression | 5 |
| Fatigue | 5 |
*Safety analysis was conducted in 12 patients (7 with POMC or LEPR deficiency and 5 with BBS).1
†Includes skin hyperpigmentation, ephelides, nail pigmentation, pigmentation lip, skin discoloration, gingival hyperpigmentation.1
‡Includes injection site bruising, pruritus, discoloration, erythema, induration, edema, pain, urticaria.1
‖Includes new melanocyte nevus formation, increased melanocyte nevus size, and darkening of pre-existing melanocytic nevus.1
¶Forty three patients were treated with at least 1 dose of IMCIVREE; 1 patient initially randomized to placebo withdrew from the study prior to receiving IMCIVREE and is not included.1
#Includes skin hyperpigmentation, hair color changes, melanoderma.1
** Includes injection site erythema, pruritus, induration, pain, bruising, edema, reaction, hemorrhage, irritation, mass.1
††n=20 male patients.1
AEs were generally mild and transient2,3
In the clinical trial for patients 2 to <6 years of age1,4,5:
- Reported incidences of nausea and vomiting primarily occurred within the first month of treatment. Vomiting was observed at a higher incidence in the study for patients 2 to <6 years of age compared with other IMCIVREE studies in patients 6 years of age and older
- Nearly all vomiting events were mild, none were serious, and all resolved
In the clinical trial for patients 6 years of age and older:
- Reported incidences of nausea and vomiting primarily occurred within the first month of treatment, then sharply declined after 4 weeks5
- Nearly all nausea or vomiting events were mild, none were serious, and all typically resolved within a few days5,6
- Nausea and vomiting should be managed by dose titration and standard care1
- The safety of IMCIVREE has been evaluated in >700 patients over 10+ years7
AE=adverse event.
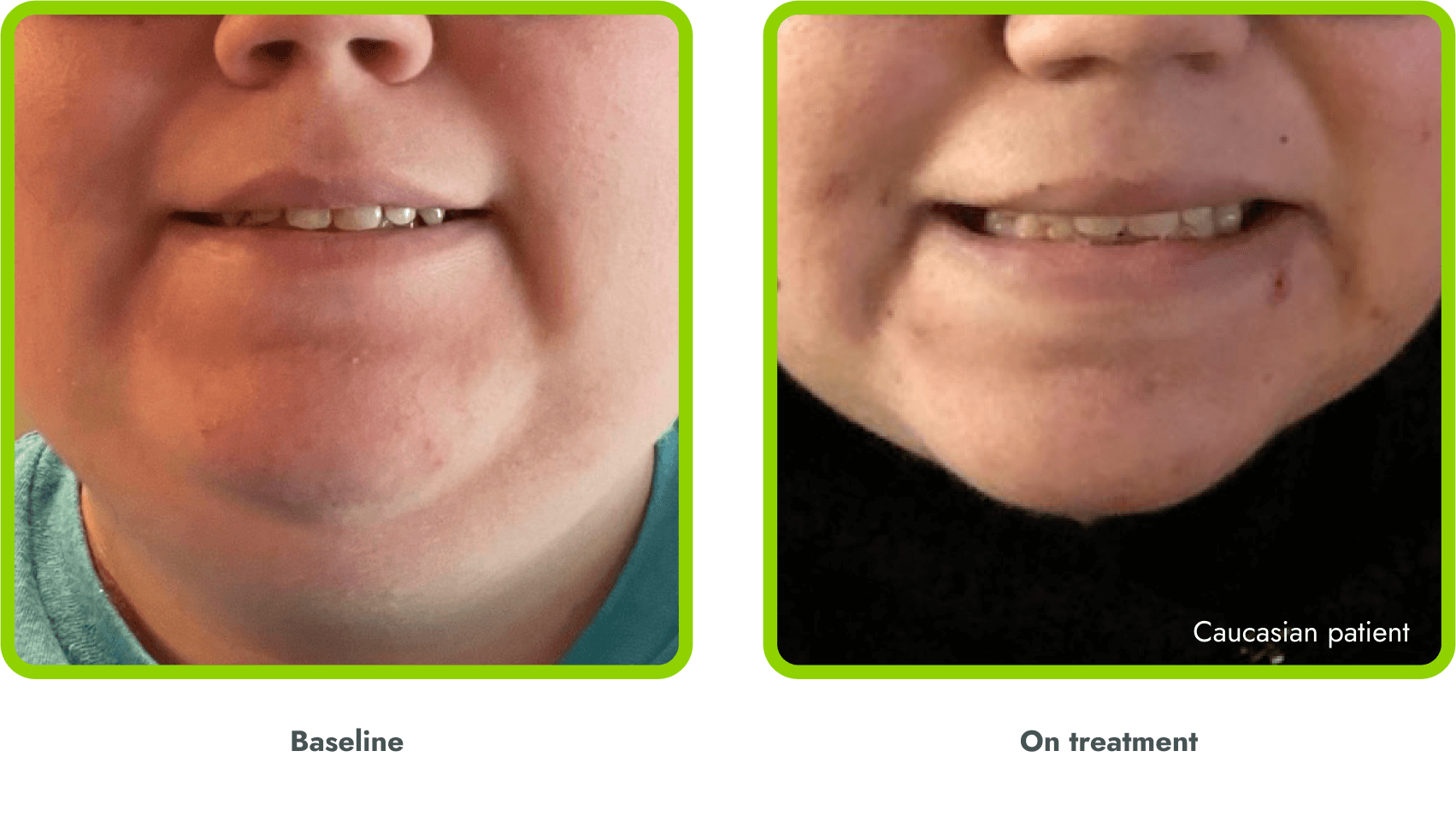
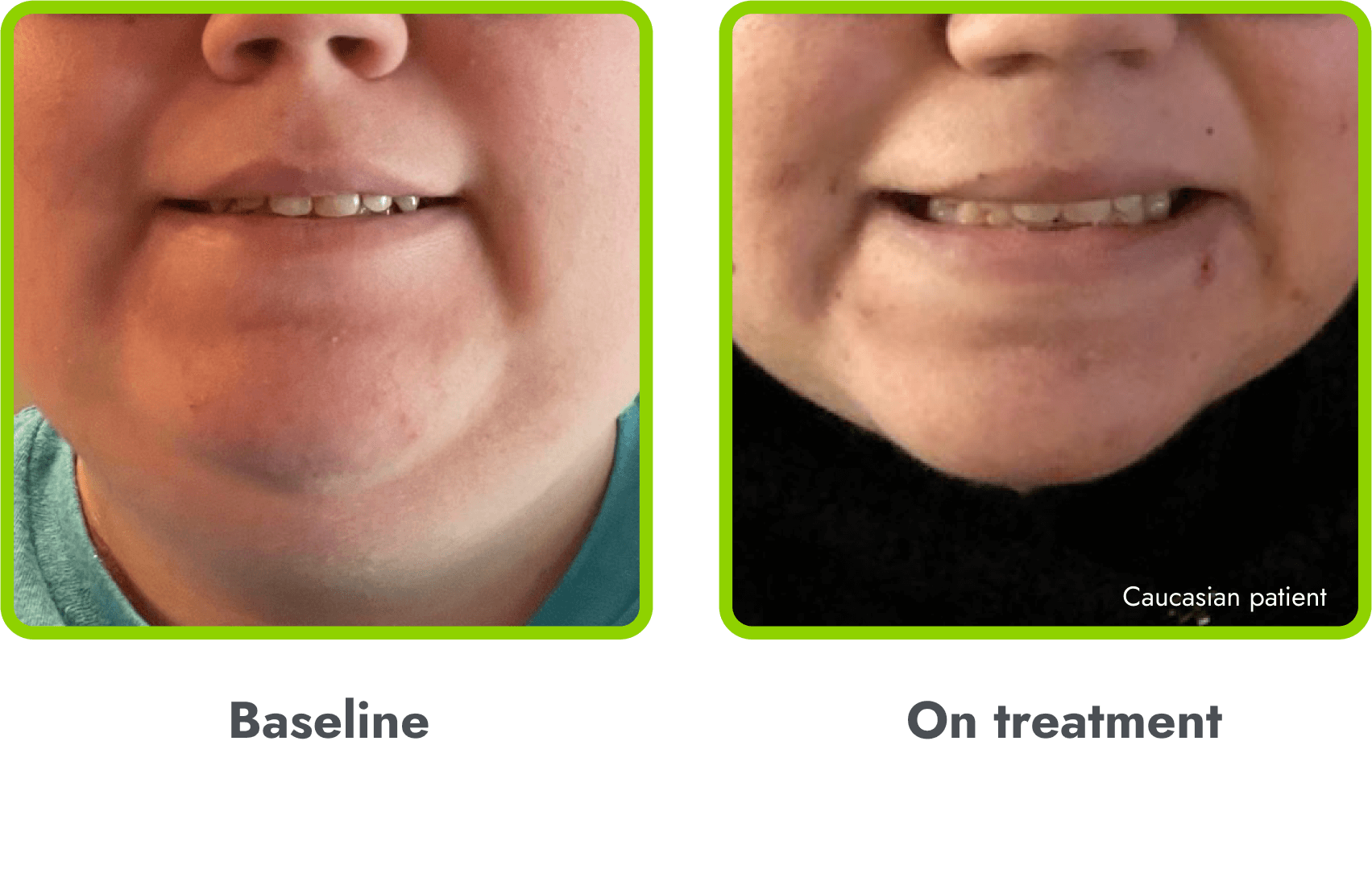
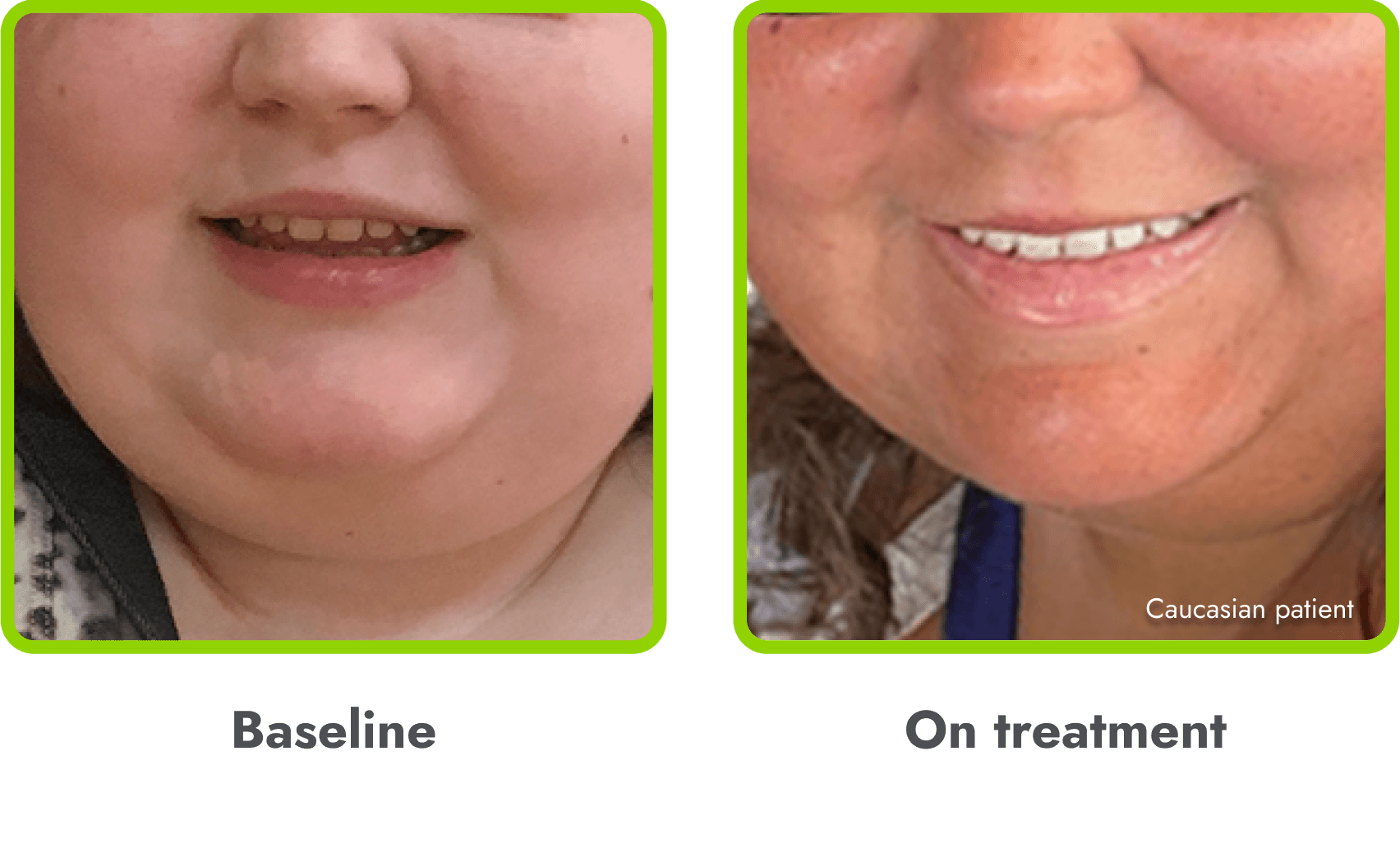
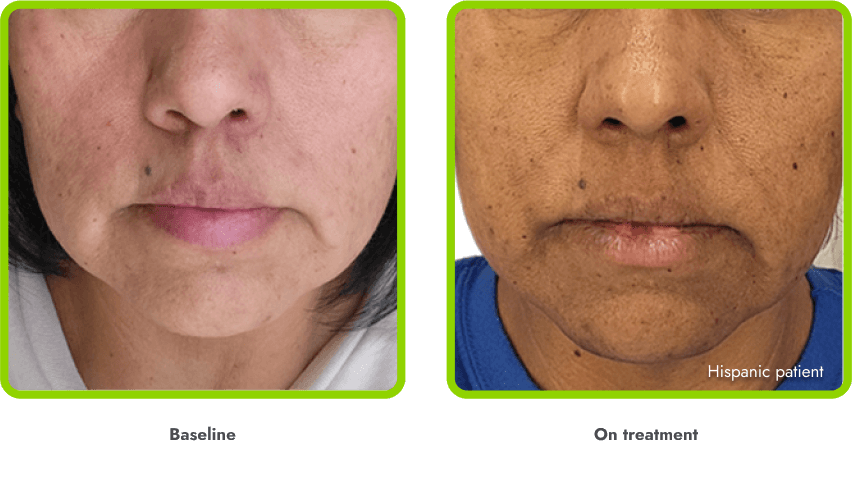
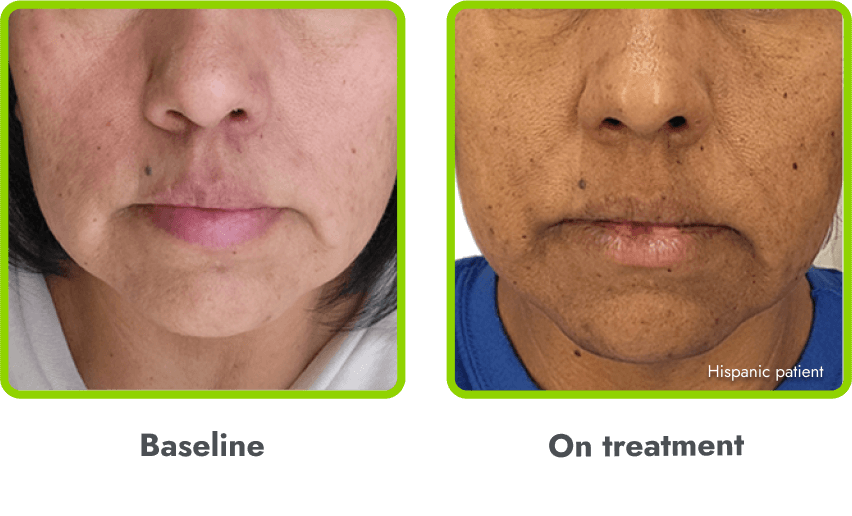
Hyperpigmentation is common1,8
- IMCIVREE is an MC4R agonist, but has some residual activity at the MC1R which commonly leads to hyperpigmentation3
- The activation of the MC1 receptor leads to accumulation of melanin which leads to hyperpigmentation4,9
- The degree of hyperpigmentation is highly variable10
- In clinical trials, measures of hyperpigmentation increased throughout the dose escalation period, and generally plateaued in the initial months of treatment1,4
- Hyperpigmentation is reversible after treatment discontinuation2
- If hyperpigmentation is a concern, assess patient response to treatment to optimize tolerability and efficacy as you would with other adverse events
Managing adverse events with IMCIVREE
Hear physicians, patients, and caregivers share their experiences with IMCIVREE
AE=adverse event; MC4R=melanocortin-4 receptor; MC1R=melanocortin-1 receptor
References: 1. IMCIVREE [prescribing information]. Boston, MA. Rhythm Pharmaceuticals, Inc. 2. Data on file. Rhythm Pharmaceuticals, Inc. Boston, MA. 3. Collet TH, Dubern B, Mokrosinski J, et al. Evaluation of a melanocortin-4 receptor (MC4R) agonist (Setmelanotide) in MC4R deficiency. Molecular Metabolism. 2017;6(10):1321-1329. doi:10.1016/j.molmet.2017.06.015. 4. Haqq AM et al. Lancet Diabetes Endocrinol. 2022;10(12):859-868. doi:10.1016/S2213-8587(22)00277-7. Supplemental appendix available at: https://www.thelancet.com/journals/landia/article/PIIS2213-8587(22)00277-7/fulltext. 5. Data on file. Rhythm Pharmaceuticals, Inc. Boston, MA. 6. Argente J et al. Endocrine Society Annual Meeting. Poster ODP606. June 11-14, 2022. 7. U.S. National Library of Medicine. Identifier: setmelanotide. ClinicalTrials.gov. Accessed January 15, 2025. 8. Argente J et al. The Pediatric Endocrine Society Annual Meeting. Poster 155. April 28-May 1, 2022. 9. Wolf Horrell EM, Boulanger MC, D'Orazio JA. Melanocortin 1 Receptor: Structure, Function, and Regulation. Front Genet. 2016 May 31;7:95. doi: 10.3389/fgene.2016.00095. 10. Kanti V, Puder L, Jahnke I, et al. A Melanocortin-4 Receptor Agonist Induces Skin and Hair Pigmentation in Patients with Monogenic Mutations in the Leptin-Melanocortin Pathway. Skin Pharmacol Physiol. 2021;34(6):307-316. doi:10.1159/000516282.