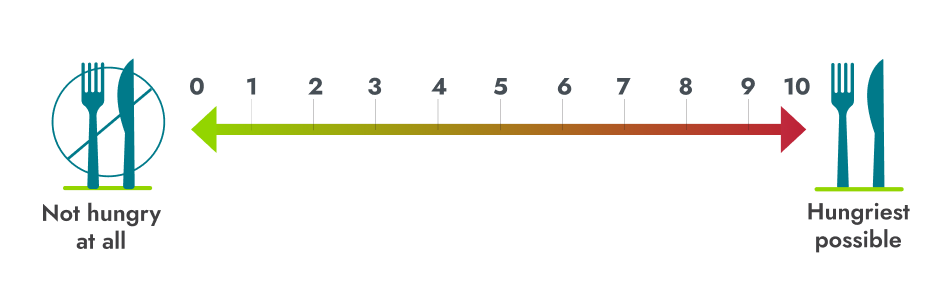In patients ≥12 years of age with BBS,
IMCIVREE delivered early, significant, and sustained hunger reduction1
Hunger scores in the 14-week placebo-controlled and 52-week open-label periods1-3*
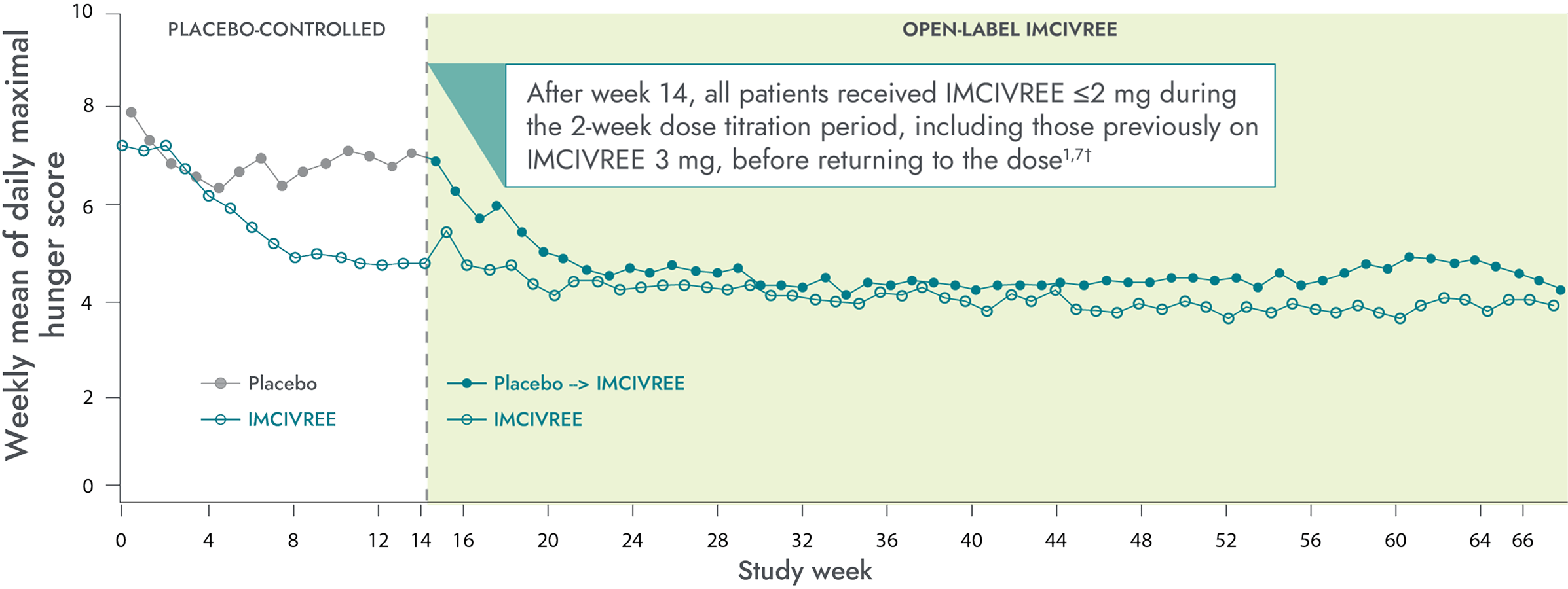
Patients who switched from placebo to IMCIVREE experienced a rapid reduction in hunger, matching that of patients initially assigned to IMCIVREE8
*Patients ≥12 years of age who were able to self-report their hunger (n=14) recorded their daily maximal hunger in a diary, which was then assessed by the Daily Hunger Questionnaire Item 2. Hunger was scored on an 11-point scale from 0 (“not hungry at all”) to 10 (“hungriest possible”).2
†During the placebo-controlled period, dose titration to a fixed dose of IMCIVREE 3 mg given subcutaneously once daily was performed during the first 2 weeks of both the placebo-controlled and open-label periods to maintain blinding.2

Before IMCIVREE, I didn’t realize how much time I spent focusing on food, and how much that was affecting my day-to-day and the other things I could be accomplishing.
— Kathryn, who is living with BBS

The change in Reed’s hunger has cascaded into many positive life changes for all of us. There's less agitation and anxiety over hunger or family meals. This is simple normalcy for many families, but for us, they’re moments I’ll never take for granted.
— Kat, caregiver of a child living with BBS

He is no longer digging through the fridge or garbage, so we do not lock them anymore. He isn’t asking for food constantly between meals and snacks, and I sometimes find myself realizing it’s been a few hours and asking him if he’s ready for a snack.
— Rachel, caregiver of a child living with BBS

Patients and families living with BBS often express such gratitude and relief when started on IMCIVREE and see the weight trajectories and hyperphagia start to improve. To have a medication that can support correcting the biology that is dysregulated and to see positive outcomes provides hope.
— Alaina Vidmar, Pediatric Endocrinologist

These are patients that nothing else has ever worked. So to see them so happy about having something that's finally helping is amazing. Now all they talk about is, ’I'm not hungry all the time. I'm not seeking out food all the time. I'm able to do other things.‘ That shift from the number on the scale to the quality of life, I really love that.
— Christy Davis, Obesity and Weight Management Specialist
References:1. Haqq AM et al. Lancet Diabetes Endocrinol. 2022;10(12):859-868. doi:10.1016/S2213-8587(22)00277-7. Supplemental appendix available at: https://www.thelancet.com/journals/landia/article/PIIS2213-8587(22)00277-7/fulltext.2. IMCIVREE [prescribing information]. Boston, MA. Rhythm Pharmaceuticals, Inc.3. Data on file. Rhythm Pharmaceuticals, Inc. Boston, MA.