How did IMCIVREE help reduce measures of weight in the clinical trials?
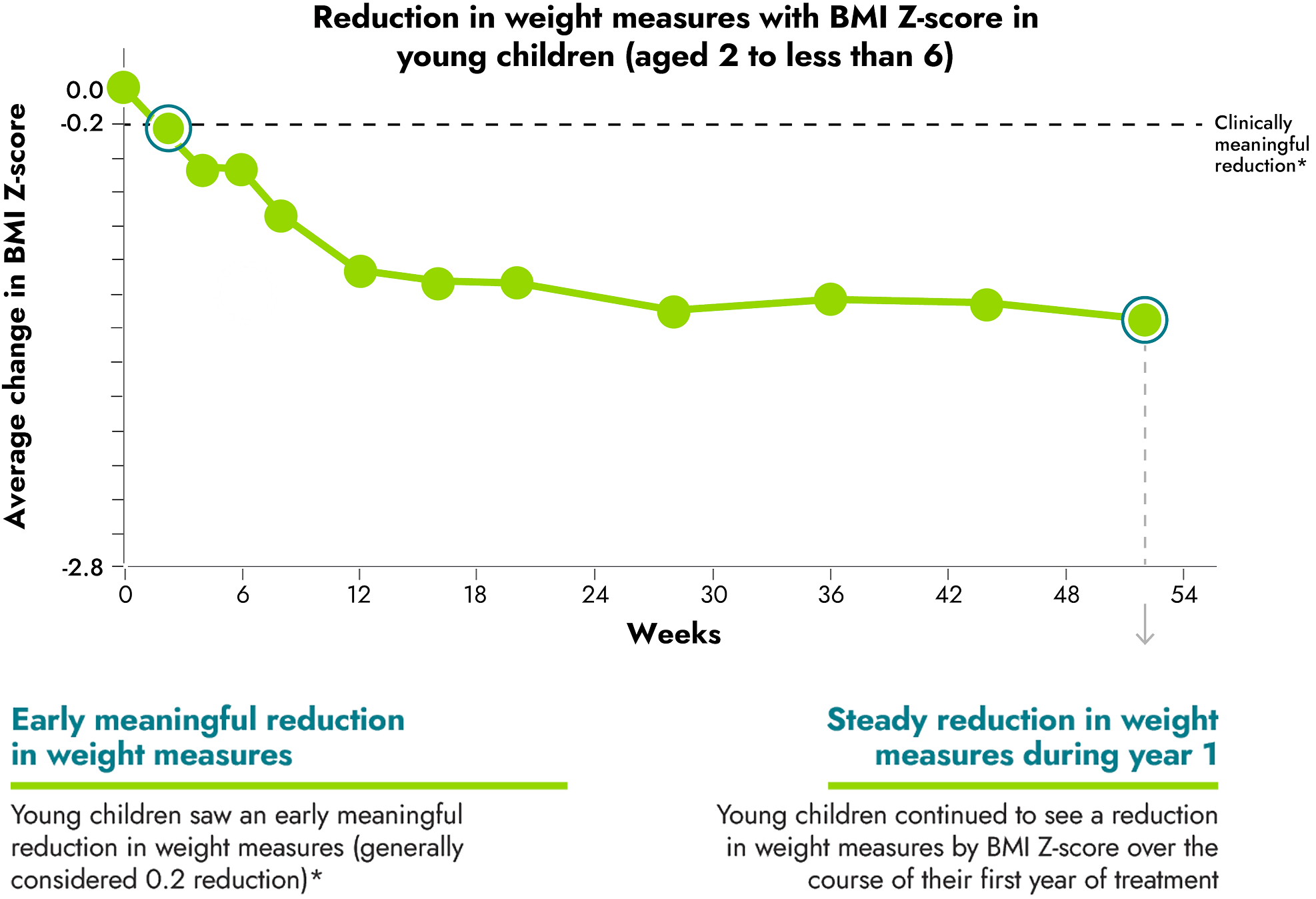
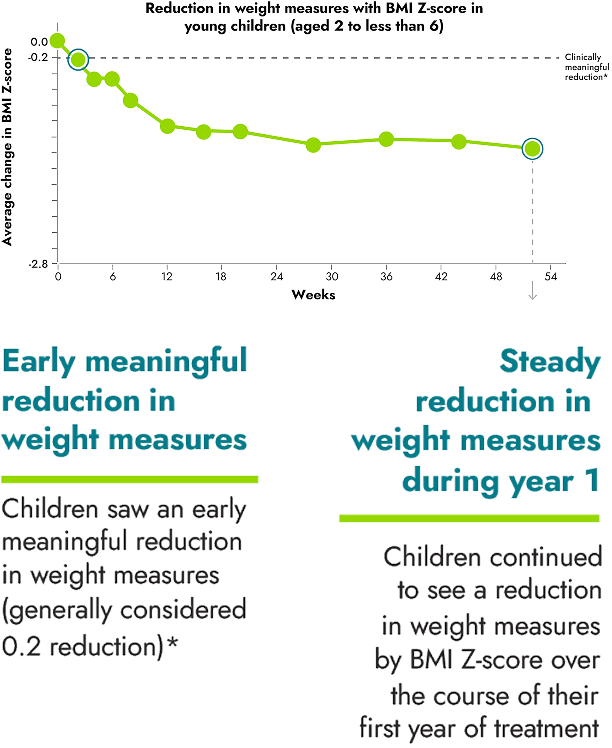
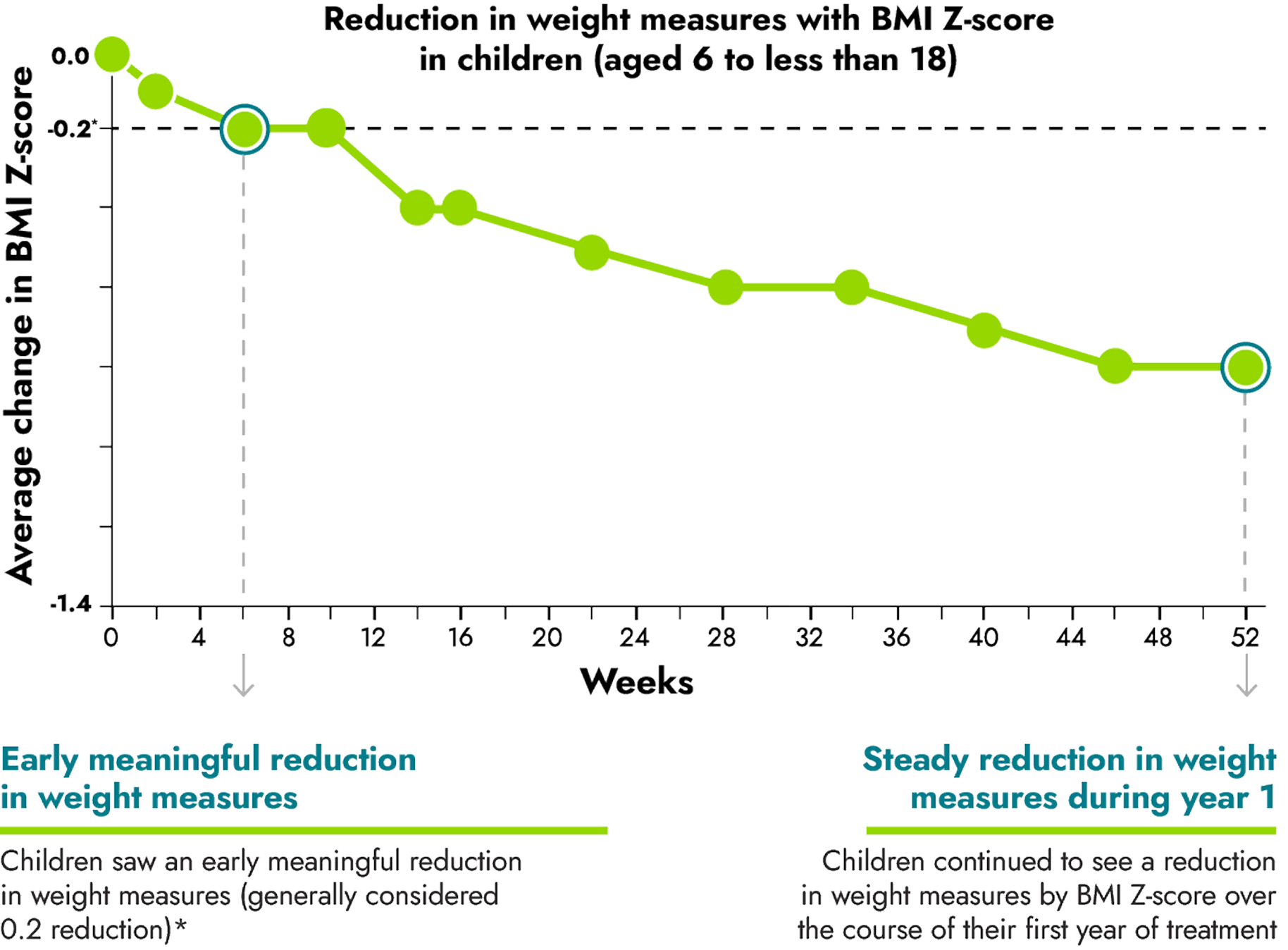
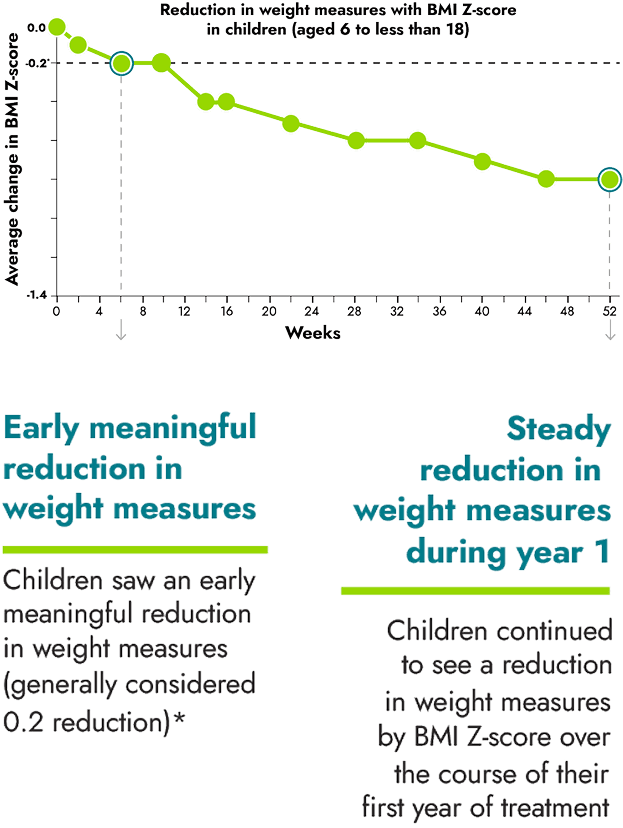
IMCIVREE helped children reach steady and long-lasting reduction in weight measures
*A clinically meaningful reduction is generally defined as a reduction of greater than or equal to 0.2 in BMI Z-score.
16 children between the ages of 6 to less than 18 were evaluated as part of the study.
A Body Mass Index, or BMI, Z-score was used to measure the reduction in BMI in children. BMI Z-scores are reliable measures of weight in children who are still growing because they take into account height, age, and gender.
No change to diet or exercise: In the clinical trial, people were not required to change their diet or exercise routine
IMCIVREE is the first and only treatment to significantly reduce BMI Z-score in children with obesity due to BBS
Some people chose to continue taking IMCIVREE in a separate long-term clinical trial. After receiving 2 years of treatment, their results were analyzed.
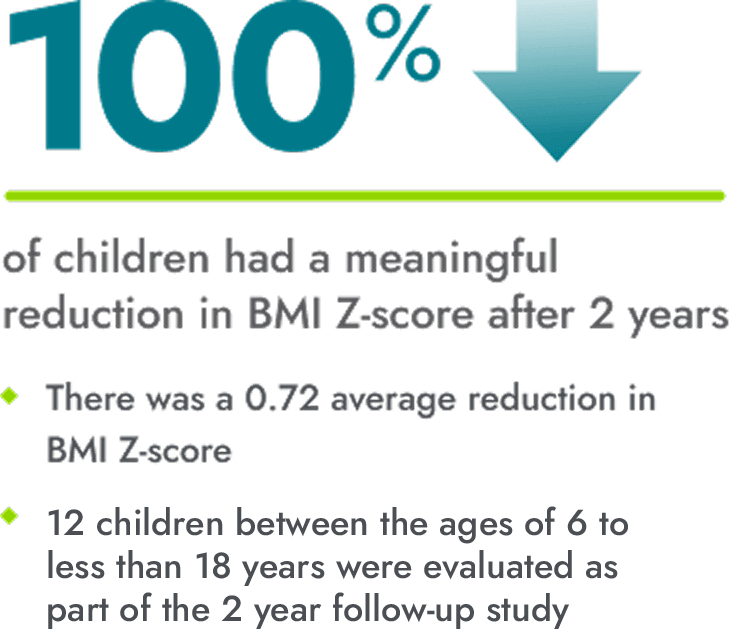
After 2 years of treatment, IMCIVREE helped children maintain meaningful reductions in BMI Z-score
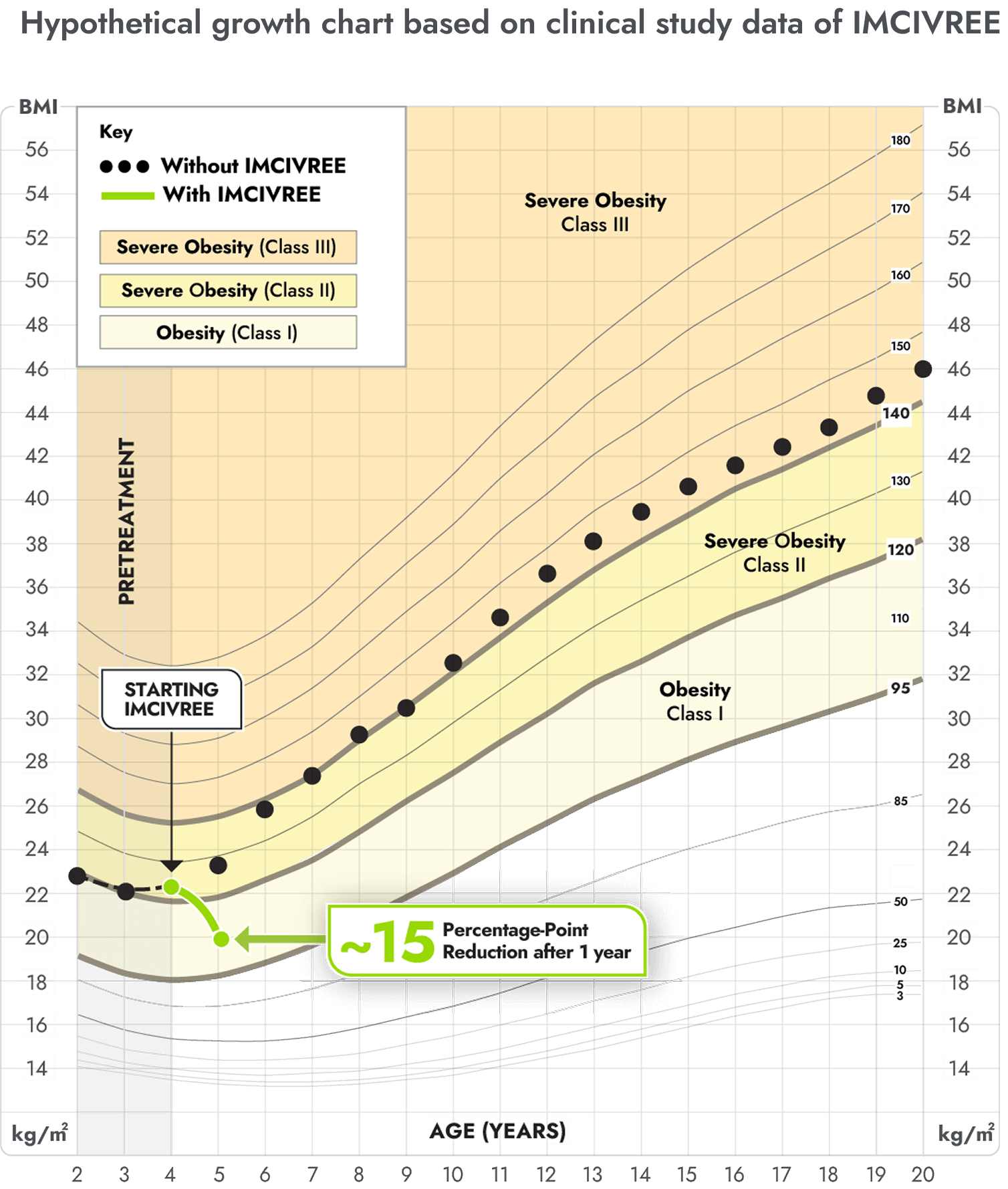
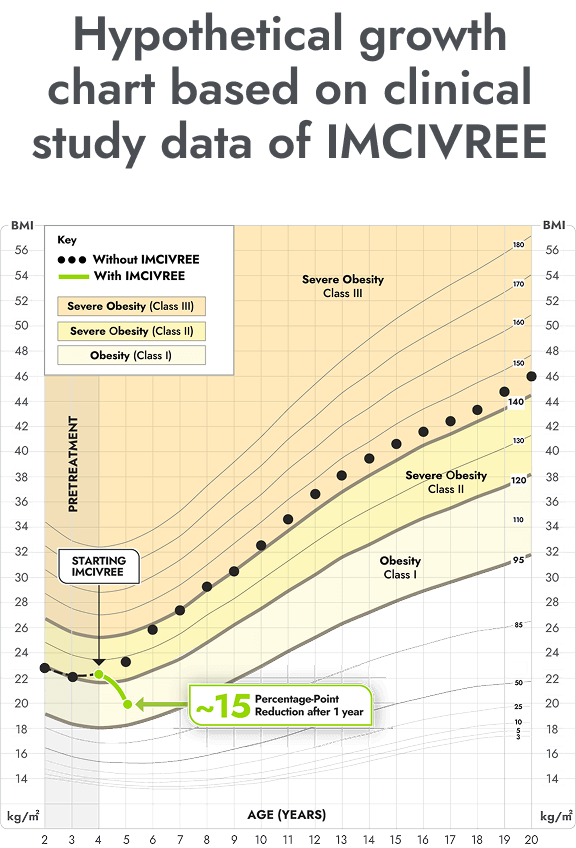
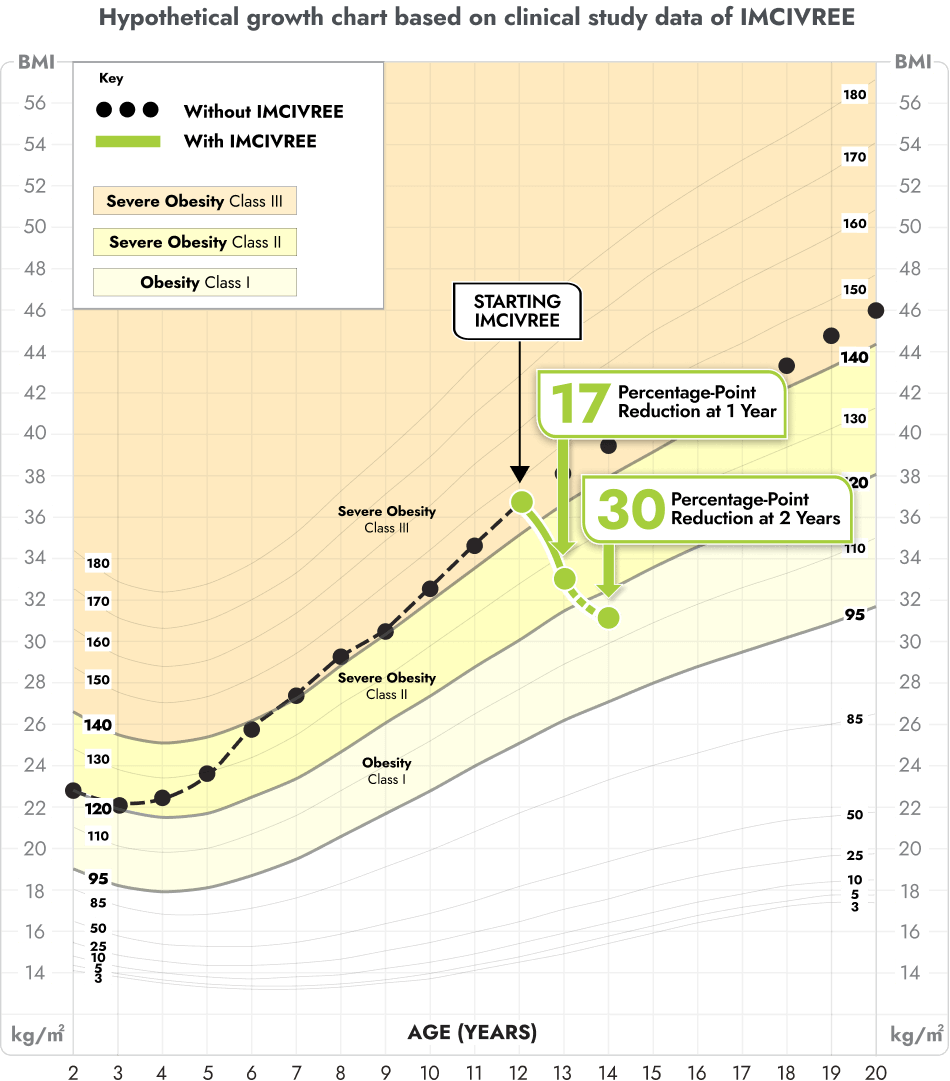
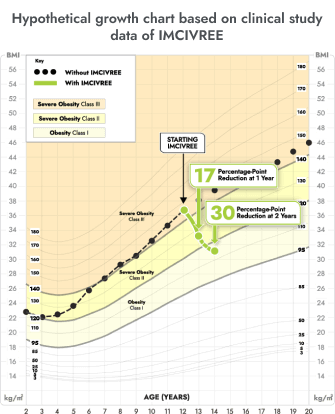
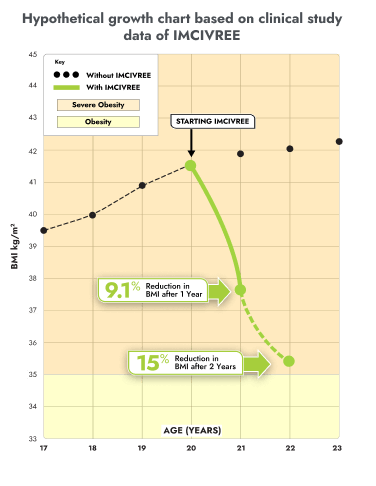
IMCIVREE reduced the severity of obesity in children living with BBS
You may be more familiar with viewing a child's growth as a percentile on a chart from the doctor. These same charts can be used for BMI. This growth chart is a hypothetical representation of what a 12 year-old female with BBS who is taking IMCIVREE may experience in BMI reduction after 1 and 2 years, based on results from the clinical study.
Figure modeled after Gulati AK, Kaplan DW, Daniels SR. Clinical tracking of severely obese children: a new growth chart. Pediatrics. 2012;130(6):1136-1140.
Not an actual patient. Growth chart is based on females 2 to 20 years of age and is for illustrative purposes only.
After 1 and 2 years on IMCIVREE, she would see a 17 percentage-point and 30 percentage-point reduction of the BMI 95th percentile, respectively.
With IMCIVREE, her weight-gain trajectory drops down to obesity (class 1). Without IMCIVREE, she would likely remain in the severe obesity (class 3 category) as she ages.
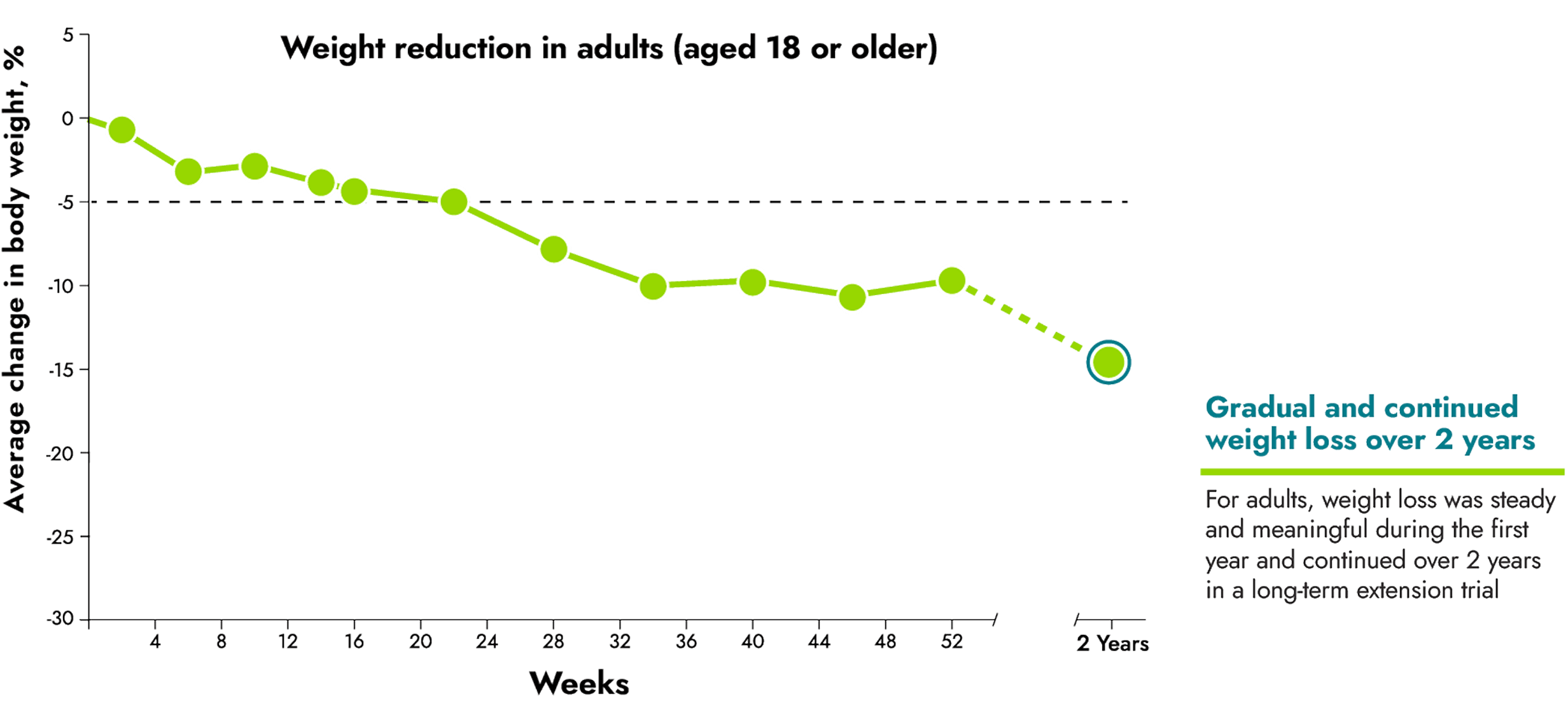
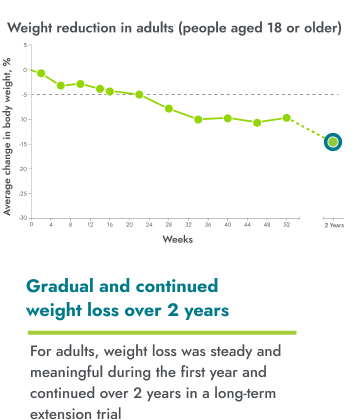
IMCIVREE helped adults reach steady and long-lasting weight reduction
IMCIVREE reduced body weight over the course of the 1-year clinical trial. At the end of a clinical trial for IMCIVREE, 19 people continued in a long-term study. 6 of these people were adults. People are being assessed every 3 months until the end of the study (up to 5 years or study withdrawal).
15 adults aged 18 years or older were evaluated as part of the study.
No change to diet or exercise: In the clinical trial, people were not required to change their diet or exercise routine
IMCIVREE reduced weight early and continuously over the course of 2 years of treatment
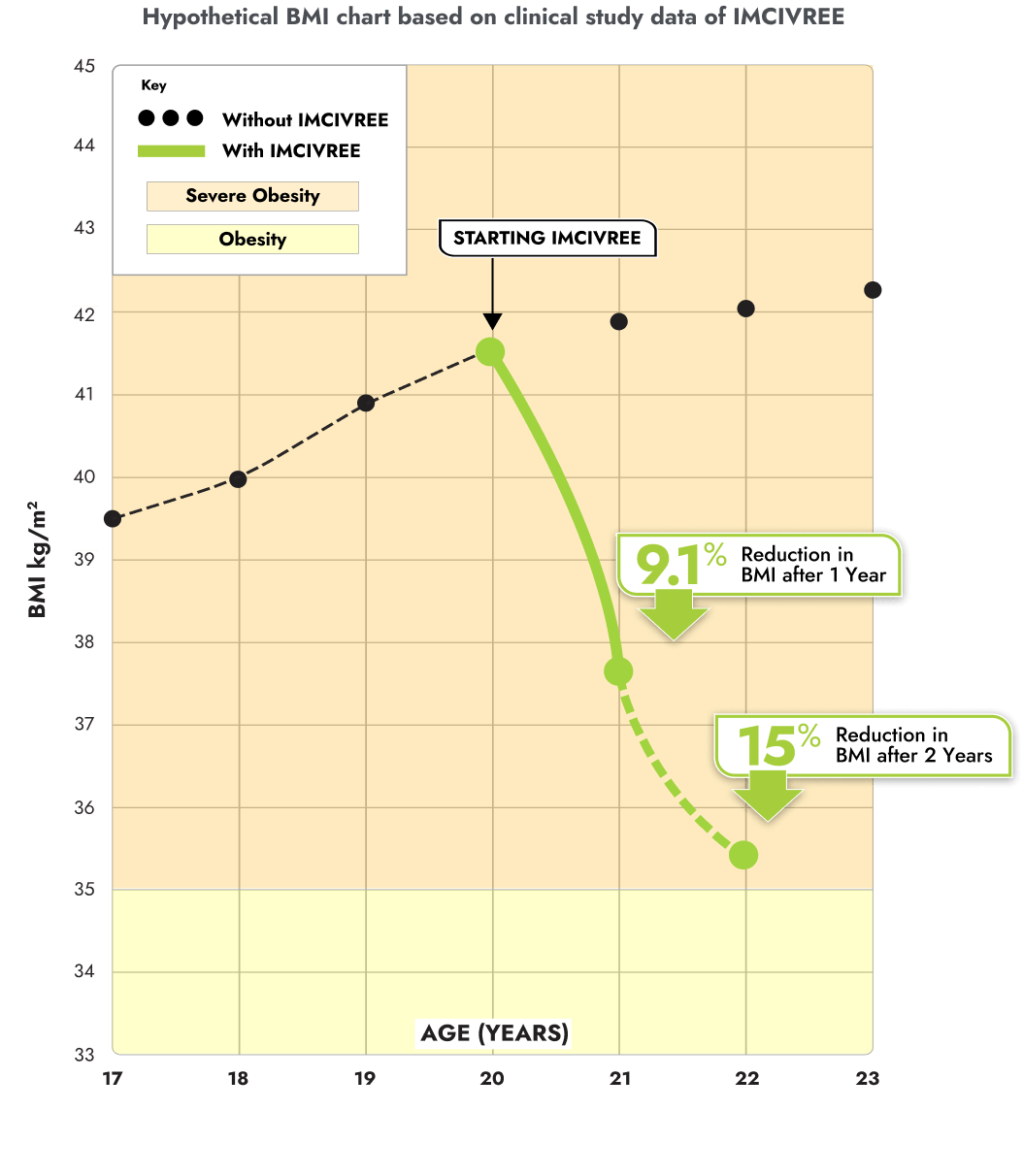
IMCIVREE reduced the severity of obesity in adults living with BBS
This BMI chart is a hypothetical representation of the BMI reduction an adult with BBS may experience after 1 and 2 years of treatment on IMCIVREE, based on data from the clinical trial.
IMCIVREE has mainly helped me lose and control my weight. I know I couldn’t have done it without this medication.
— Adult patient enrolled in the IMCIVREE clinical trial
Individual results may vary.
IMCIVREE provided reduction in hunger scores early and continuously throughout treatment in people 12 years and older
The effect of IMCIVREE on reducing hunger was studied in people 12 years and older living with BBS who could self-report their hunger.
- They completed a questionnaire every day for 1 year to determine changes in their hunger
- People scored their hunger on a daily basis using a scale from 0 to 10
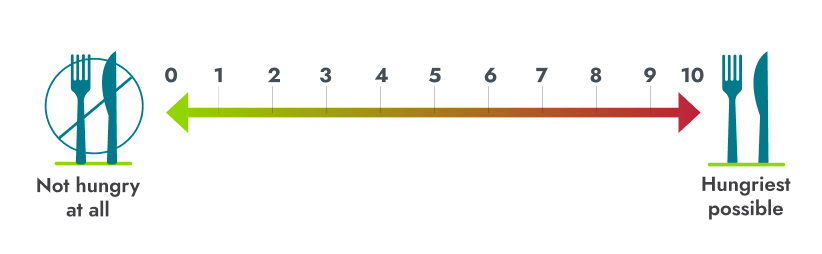
IMCIVREE reduced the most severe feelings of hunger in people 12 years and older
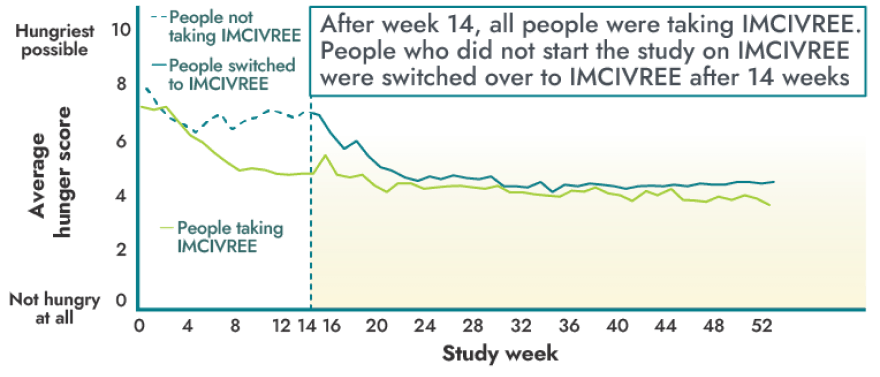
- 14 people were studied.
A majority of people 12 years and older reported a reduction in hunger within weeks of starting IMCIVREE

Before IMCIVREE, I didn’t realize how much time I spent focusing on food, and how much that was affecting my day-to-day and the other things I could be accomplishing.
— Kathryn, a person living with BBS
The change in Reed's hunger has cascaded into many positive life changes for all of us. There's less agitation and anxiety over hunger or family meals. This is simple normalcy for many families, but for us, they're moments I'll never take for granted.
— Kat, caregiver of a child living with BBS


He is no longer digging through the fridge or garbage, so we do not lock them anymore. He isn’t asking for food constantly between meals and snacks, and I sometimes find myself realizing it’s been a few hours and asking him if he’s ready for a snack.
— Rachel, caregiver of a child living with BBS
Individual results may vary.
How did people with BBS and obesity report changes to their health-related quality of life?
As part of this study, people with BBS were asked to evaluate aspects of their quality of life.
For children, questions in the survey measured:
- Physical ability in daily activities
- Emotional state
- Social needs
- Ability to perform at school
For adults, the questions in the survey measured:
- Physical ability in daily activities
- Self-esteem
- Sexual life
- Feelings of distress in public
- Ability to perform at work
Higher survey scores indicated improvement to daily life. Lower scores indicated a decrease.
On average, people reported higher scores after 1 year of taking IMCIVREE.
Although general improvements were measured, there were a limited number of people to make clear conclusions. The trial was also not set up to determine whether these changes were because of IMCIVREE.
The impact of IMCIVREE
Learn more about the impact of IMCIVREE from healthcare providers and people on treatment